Articles
Battery Charge Regulation
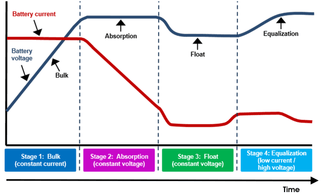
Overcharging a battery raises the temperature of the electrolyte,...
Read more
Battery Lifetime, Efficiency and Care
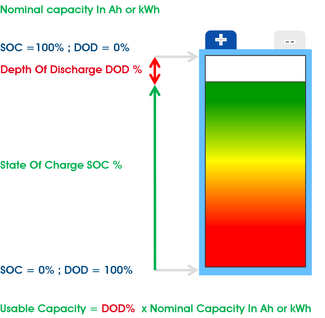
Battery life is measured in number of discharge/charge cycles rather...
Read more